1/24/2014
The wheels of progress and change often move slowly, and never more so than in a bureaucracy. The Food Safety Modernization Act (FSMA) representing a major shift in food safety, probably the biggest in the last 70 years, was signed into law early in 2011. Today, in early 2014, many aspects of the FSMA are still being discussed by the Food and Drug Administration and Congress.Just how will all those new elements be enforced and who will need to comply? Those are just two of the big questions still being decided, often on a guideline by guideline basis, in Washington and communities just like yours.
Following the FDA Process
The FDA, in conjunction with Congress, issues a number of rulings and guidelines, as set out in the Administrative Procedure Act, yet another law, that governs how the federal government can propose and establish regulations. Under this act, the FDA can and does hold public meetings to provide all interested parties an opportunity to come and raise issues or concerns about the proposed legislation. In some cases, but not all, feedback is factored into the enforcement or ruling, in effect changing the way in which the legislation is applied.
- FDA proposes a rule and requests comments - Also known as a Notice of Proposed Rule Making (NPRM), it’s published in the Federal Register (FR) so that the public can view and send comments, typically between 30 and 90 days.
- FDA considers all comments and issues a final rule - Any significant comments are discussed and sometimes written into the review. Sometimes those comments result in a rule revision. The final rule is also published in the FR and in the FDA’s official docket on Regulations.gov.
- Companies comply with the rule based on the identified “Effective Date” - The amount of time can vary and the sometimes additional provisions or accommodations are made for various industries such as farms, small business, etc.
Actual Case Study
One such example, starting back in January 2013, regarded rules about produce safety and preventive controls for human food. One aspect addressed science-based standards and the other aspect addressed safety standards for food facilities. In both cases, the FDA made unprecedented steps to engage all stakeholders, traveling across the globe to meet with industry, farms of various types and sizes, and food producers of all kinds. Because of the valuable input received during those interviews, it quickly became clear that the language would have to be revised in order to achieve the goal of the legislation while minimizing undue burden to local farmers and growers. You can reference the Dec 19th publication where the results of this inquiry are detailed. Be on the lookout for the revised ruling due out in summer 2014.
Latest FDA request for input
One of the currently proposed rules, published Dec 24, 2013, for the “Mitigation Strategies to Protect Food Against Intentional Adulteration” is currently posted and available for comments. Comments to the proposed rule are due by March 31, 2014. Under this proposition, the largest food businesses would have to have a written food defense plan addressing significant vulnerabilities in a food operation. The FDA is proposing tiered compliance dates, recognizing that small and very small businesses may need more time to comply with this requirement. This rule, as currently written, would apply to all foreign and domestic facilities that manufacture, process, pack or hold food and are required to register as a food facility under section 415 of the FD&C Act.
Opportunity and Challenge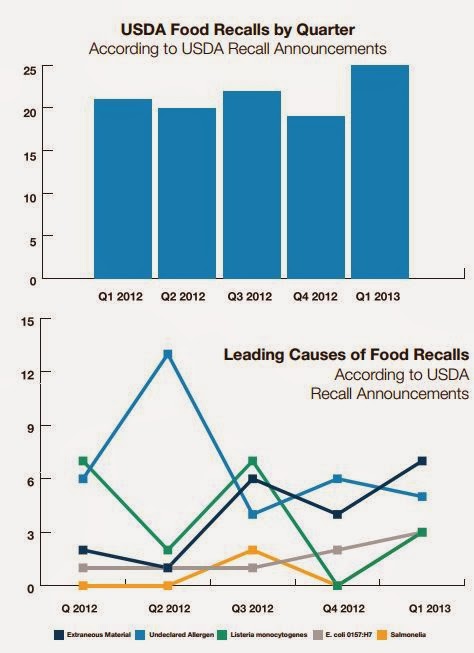
A recent Food Manufacturing article on the FSMA rightly identified it as an opportunity and a challenge. The FSMA moves from responding to food safety breakdowns to preventing breakdowns in food safety, in an attempt to minimize the increasing number of recalls and outbreaks on the rise in recent years. The challenges, particularly for the smaller food processor and local farmer, offset by tiered compliance dates, may be insufficient to relieve the pressure of meeting all of the FSMA requirements.
The opportunity is for those who can get ahead of the curve, be better informed and aware of changes, and be at work or have already achieved accreditation to Good Manufacturing Practices (GMP) and food safety such as SQF and HACCP.
What do you know about FSMA? Are you ready? Share Your Story in the comments.
Since 1991, MMTC has assisted Michigan’s small and medium-sized businesses compete and grow. Through personalized services fitted to meet the needs of clients, we develop more effective business leaders, drive product and process innovation, promote company-wide operational excellence and foster creative strategies for business growth and greater profitability. Find us at www.mmtc.org.
Categories: Food Processing